2.12.1.3 Case III: Simultaneous presence of oxygen vacancies and metal interstitials
Such a scenario is often found in ceramic oxides like TiO2, and Nb2O5.
Consider a metal oxide (MO2) with doubly charged oxygen vacancies and metal ion interstitials. The corresponding defect reaction is
Assuming 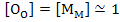 , the defect equilibrium can be written as , the defect equilibrium can be written as
| [VO••] ne2 pO21/2=K1 |
(2.37) |
[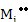 ] ] 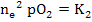 |
(2.38) |
According to the electrical neutrality condition
| ne = 2[VO••] + 2[Mi••] |
(2.39) |
Two limiting cases can be considered:
When [V0••] >>[Mi••]
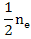 = [VO••] = = [VO••] = 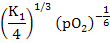 |
(2.40) |
And
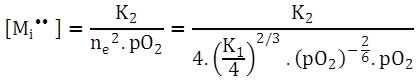 |
(2.41) |
i.e.
[Mi••] 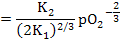 |
(2.42) |
As you can see, under such conditions, [Mi••] decreases more rapidly with increasing pO2. This is commonly observed in TiO2 and Nb2O5 where [V0••] can be 1010 times higher than [Mi••].
When [Mi••] >>[V0••]
Following similar exercise as above, we can calculate
[Mi••] = 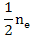 = = 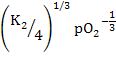 |
(2.43) |
and
[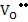 ] = ] = 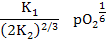 |
(2.44) |
Here, [V0••] increases with increasing pO2 while keep decreasing with increasing pO2 but at a different rate.
Figure 2. 5 Defect concentration vs pO2 in an oxygen deficient oxide with oxygen vacancy as dominating defect |
|