2.12.1.2 Case II: If interstitial metal or metal excess is present
Here, the defect reaction will be
The equilibrium constant can be written as
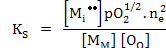 |
(2.34) |
Both [MM] and [OO] can be assumed to be ~1 if [Mi••] << [MM] and [OO].
According to the electrical neutrality condition
Thus
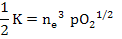 |
OR |
ne
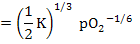 |
(2.36) |
The plot will be similar to that of the above case.
|