|
2.12.2 Metal Deficient Oxides
Now we turn towards the case of MO type oxides with deficient of metal which can be reflected either by metal vacancies or oxygen interstitials or presence of both. Here we do analysis only for metal vacancies while other two cases can be done in a similar fashion as shown in previous paragraph.
For MO oxide, assuming complete ionization of vacancies, we can write
whose equilibrium constant will be
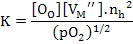 |
(2.45) |
If 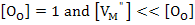 then then
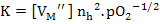 |
(2.46) |
According to the electrical neutrality condition
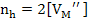 |
(2.47) |
Again, the concentration of defects is proportional to pO21/6.
One can do similar exercise for the cases when oxygen interstitial is the main defect and also when there is mixed presence of metal vacancies and oxygen interstitials. This is left to the readers to perform themselves.
|