2.12.1.1 Case I: When oxygen vacancies are dominant defects
Here, the defect reaction can be expressed as
The reaction constant will be
K = [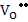 ] ] 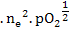 |
(2.29) |
Here,we assume that [OO] = 1. From the above reaction, to maintain the electrical neutrality, ne = 2 [VO••].
Thus
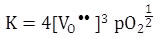 |
(2.30) |
or
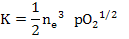 |
(2.31) |
or
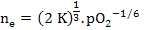 |
(2.32) |
and
[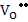 ] = ] = 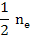 |
(2.33) |
The relationship between the defect concentration and pO2 can be seen in the schematic figure where defect concentration varies as pO2-1/6. This makes sense because concentration of vacancies will go down as we supply more oxygen to the material.
| Figure 2. 4 Defect concentration vs pO2 in an oxygen deficient oxide with oxygen vacancy as dominating defect |
|