|
3.2.4 Temperature Dependence of Diffusivity
Now, equation (3.9) can further be modified by replacing the jump frequency, Γ , which, by Boltzman statistics, is defined as
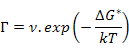 |
(3.10) |
where ν is the vibration frequency in s-1, ΔG* is the activation energy of migration in J and k is Boltzmann Constant (J/K).
Further, ΔG* can be written as
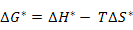 |
(3.11) |
where ΔH* is the enthalpy of migration and ΔS* is the associated entropy change. Now, substitution of equation (3.10) in equation (3.11) leads to
or
where pre-exponential factor 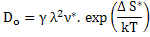 . .
Equation (3.12) explains the thermally activated nature of diffusivity showing an exponential temperature dependence resulting in significant increase in the diffusivity upon increasing the temperature. |