|
3.3 Examples of Diffusion in Ceramics
3.3.1 Diffusion in lightly doped NaCl
NaCl is often used a conducting electrolyte and it a good case for proving an example. Consider the ease of NaCl containing small amounts of CdCl2 lIn such scenario, for each Cd ion, Cd occupying Na site with an extra positive charge and a sodium vacancy with one negative charge is created according to the following defect reaction:
CdCl2 → CdNa• + 2ClClx + VNa′ |
(3.13) |
In addition, NaCl will also have certain intrinsic sodium and chlorine vacancy concentration (VNa′ and VCl•) due to Schottky dissociation, depending on the temperature.
In such a scenario, the diffusivity of sodium ions is governed by vacancy diffusion and can be worked out as
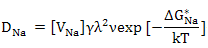 |
(3.14) |
where ΔGNa* is the migration free energy for sodium vacancies and VNa' is the sodium vacancy concentration. This diffusivity of vacancy is dependent on vacancy’s concentration which is related to the dopant concentration. However, the diffusivity dependence on temperature shows two regimes as shown in figure 3.3; low temperature extrinsic regime where vacancy concentration is independent of temperature and is determined by solute concentration and another high temperature intrinsic regime where vacancy concentration is dominated by intrinsic thermally degenerated vacancies.
| Figure 3. 3 Schematic diagram showing temperature dependence of diffusivity in ceramics |
3.3.1.1 Low Temperature Regime:
Extrinsic region is dominant where vacancy concentration is constant as it is determined by the solution concentration i.e. [VNa'] = [CdNa•] . The diffusivity is given by
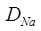 |
|
| |
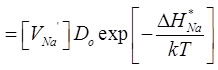 ..........................................(3.15) ..........................................(3.15) |
In this regime, diffusivity exhibits a temperature dependence of 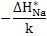 . .
3.3.1.2 High Temperature Regime:
In this regime, the vacancy concentration is governed by intrinsic defect creation mechanism with Schottky defect formation and hence, diffusivity exhibits a steeper slope with higher activation energy which include not only the energy for defect migration but also for defect creation i.e 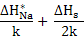
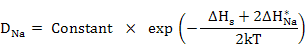 |
(3.16) |
At the point of crossover of two regimes, vacancy concentration due to dopants equals the thermally created vacancies.
|