|
2.11 Defect Equilibria in Stoichiometric Oxides
The defects which we usually consider in stoichiometric oxides are Schottky and Frenkel defects and following paragraphs so analysis for both these kinds of defects for an oxide MO.
2.11.1 Schottky Defects
Defect reaction in an oxide MO is written as
Equilibrium constant for this reaction is
KS = [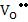 ]
[VM''] ]
[VM''] |
Here square brackets i.e. [ ] are used for concentration.
Equilibrium constant can be also be expressed as
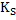 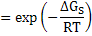 |
(2.22) |
where ΔGS is the molar free energy of defect formation and is ΔHS - TΔSS, where ΔHS is the enthalpy for defect formation and ΔSS is the entropy change which is mainly vibrational in nature and can be assumed to be constant. This leads to
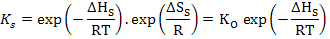 |
(2.23) |
If Schottky defects dominate, then
[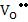 ] ] 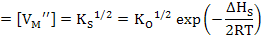 |
(2.24) |
Here, as one can see, defect concentrations are independent of pO2.
2.11.2 Frenkel defects
For an oxide MO
which leads to
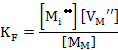 |
(2.25) |
OR
At reasonably low defect concentrations when
| [Mi••] and [VM'']
<<
MM and MM≈1 |
=
Thus
If Frenkel defects dominate, then
i.e.
| [Mi••]=[VM'']=KF1/2 |
(2.27) |
In a similar manner what we did above for Schottky defects, one can now write
[Mi••]=[VM'']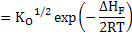 |
(2.28) |
Again we can see that the defect is independent of pO2. |