2.10 Defect Equilibria
2.10.1 Thermodynamics of Defect Reactions
A defect reaction can be treated like a chemical reaction allowing us to relate the thermodynamic variables like pO2 temperature to the free energy change or enthalpy change which can be determined using experimental techniques. This allows us to establish, for example, an equilibrium diagram between defect concentration and pO2, helping us to identify various regions which may be useful under practical conditions. For detailed chemical thermodynamics, you should refer to the appropriate subject or books.
So, if a chemical system consists of n1 + n2 + ---- +ni moles of constituents 1, 2, 3, ………..,i, the partial molar free energy of Ith constituents is given as
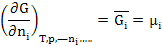 |
(2.16) |
Then, according to the Gibbs Duhem equation, at equilibrium
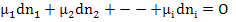 |
(2.17) |
In a chemical reaction
Free energy change can be written as
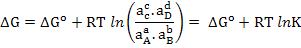 |
(2.18) |
where ΔGo 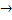 . Free energy change is standard state i. e. at unit activities. . Free energy change is standard state i. e. at unit activities.
At equilibrium, ΔGo = 0, , hence
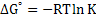 |
(2.19) |
where, K is equilibrium or reaction constant and 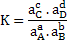 . .
In addition, free energy can be expressed as
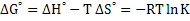 |
(2.20) |
where
which leads to K = K0exp (-ΔH0/RT), where K0 = ΔS0 and R is the gas constant.
Alternatively,
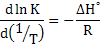 |
(2.21) |
This is an important outcome as it shows that we can treat the defects in a solid as solutes in a solvent. |