2.7 Concentration of Intrinsic Defects
Let us consider the formation of Frenkel defects in a halide, MX, i.e.
MM+ XX 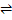 VM' + Mi•+ XX VM' + Mi•+ XX |
Change in the free energy (ΔG) upon formation of 'n' Frenkel defect pairs at an expense of ΔGf energy per pair
where ΔSC is the change in configurational entropy and is positive. Equilibrium concentration of defects is found by minimizing ΔG w. r. t. n i.e. the concentration at which free energy is minimum.
Change in entropy is given by
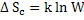 |
(2.2) |
where W is the number of ways in which defects can be arranged.
Now, as per the defect reaction shown above, number of Frenkel pairs (n) would lead to the formation equal number of interstitials (ni) as well as vacancies (nv) i.e.
Assume that total number of lattice sites = N
Number of ways to arrange the vacancies, Wv is
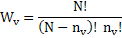 |
(2.4) |
Ways to arrange the interstitials (assuming that N lattice sites are equivalent to N interstitial sites), Wi are
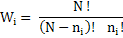 |
(2.5) |
Total number of possible configurations
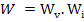 |
(2.6) |
So, entropy change will now be
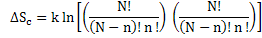 |
OR |
or
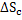 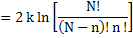 |
(2.7) |
For large values of N, Sterling’s approximant 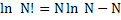 can be applied which leads to can be applied which leads to
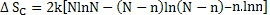 |
(2.8) |
and total free energy change is
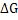 |
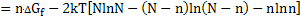 |
(2.9) |
| |
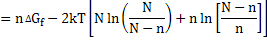 |
(2.10) |
| Figure 2.3 Equilibrium Vacancy Concentration |
Now, if vacancies were stable defects, then at certain concentration, the free energy change has to be minimum, as shown in the figure. Hence, at equilibrium, we can safely write that
Now at equilibrium,
We can also assume 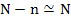 since number of vacancies is much smaller than number of lattice sites in absolute terms. since number of vacancies is much smaller than number of lattice sites in absolute terms.
This results in
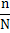 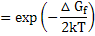 |
(2.12) |
Now we know that ΔGf = ΔHf - TΔSv where ΔH enthalpy of Frenkel defect formation and ΔSv = vibrational entropy change.
Hence Equation (2.12) further simplifies to
| |
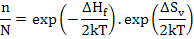 |
(2.13) |
Assuming that exp (ΔSv/2kT ) ~1 as vibrational entropy change is very small, and hence
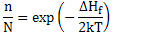 |
(2.14) |
Similarly, for Schottky defects, you can work out that
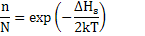 |
(2.15) |
|