|
2.19.4 Point Defects and Crystal Density in ZrO2 when doped with CaO
CaO doping in ZrO2 allows stabilization of high temperature tetragonal or cubic polymorphs of ZrO2 at room temperature as a metastable phase.
CaO doping also introduces the defects according to
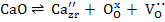 |
(1) |
OR
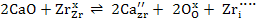 |
(2) |
From (1), oxygen vacancies formed allow ZrO2 to possess high ionic conductivity as a result cubic ZrO2 is used as electrolyte. When CaO in ZrO2 dissolves substitutionally, it has to be compensated by creation of a positive charge.
These substitutions also lead to a change in crystal density of ZrO2.
| Figure 2.10 Density of ZrO2 as a function of CaO doping |
For more case studies, readers are referred to Chapter 2 of Physical Ceramics by Chiang, Birnie and Kingery (see bibliography).
|