2.19.3 Electronic vs Ionic Compensation of Solutes
Here we will discuss which of the electronic or ionic compensation of solute incorporation in oxides is favoured and what are the conditions determining this.
In oxide semiconductors, the effectiveness of a donor or an acceptor is not only governed by their ionization energies, it is also governed by the extent of oxidation and reduction, even in case of shallow dopants with smaller ionization energies. This is due to the fact that an aliovalent impurity in an ionic compound can be charge compensated by ionic defects (ionically compensated ) or by electrons or holes (electronically compensated ) or by a combination of the two. Variables governing the extent of these are pO2, dopant concentration and temperature.
We will take the example of Nb2O5 doping in TiO2.
The defect reactions are written as
| Ionic compensation |
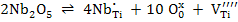 |
(1) |
| Electronic compensation |
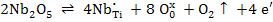 |
(2) |
Combination of the two reactions i.e. ((1) – (2)) leads to
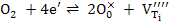 |
(3) |
Equation (3) shows that as pO2 increases, oxidation is favored and hence formation of titanium vacancies is more likely. Similarly, as the temperature reduces, oxidation is again favored.
Thus Nb doping of TiO2 tends to be compensated by VTi'''' if Nb2O5 concentration is large, pO2 is high and the temperature is low ,whereas the inverse conditions favour the electronic compensation.
In any case, the electrical neutrality condition requires that
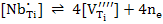 |
(4) |
Similar effects are observed in care of titanates such as BaTiO3.
|