| |
2.5.1 Oxygen Deficient Oxides
2.5.1.1 If oxygen vacancies are the dominating defects
-
Depicted by MO2-x (x is the extent of non-stoichiometry) and overall reaction as
Due to loss of oxygen, possible defect reactions would be
O0 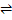 VO••+ ½ O2+ 2M'M VO••+ ½ O2+ 2M'M |
2.5.1.2 If metal interstitials are the dominating defects then,
-
Depicted as (M1+yO2 is the extent of non-stoichiometry)
-
Possible defect reactions are
-
Creation of quasi-free electrons (extra charge is represented as M’)
-
Conduction occurs due to transport of electrons
-
Typically n-type conductors.
-
Example: TiO2, ZrO2, CeO2, Nb2O5
|