| |
1.10.2 Corundum (Al2O3) Structured Compounds
M2X3 type of compounds -
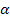 - Alumina or Sapphire (Al2O3) is the parent compound. - Alumina or Sapphire (Al2O3) is the parent compound.
-
Anions form an HCP lattice
Two-third of octahedral voids are occupied by the cations to maintain the stoichiometry. -
Coordination numbers: M: 6, X: 4.
This arrangement preserves the charge neutrality as you can also verify using bond strength formula. This can be best viewed when we look at the basal plane of (0001)-plane of the unit-cell and start filling the interstices.
Figure 1.40 Layered filling of Corundum |
One unit-cell consists of six layers of oxygen ions. A side view of the structure on plane can be seen below where you can see columns of cations along the c-axis with
2⁄3
rd filling of octahedral sites.
Figure 1.41 View of {1010} plane of Corundum |
|