| |
3.12 Fast Ion Conductors
-
Materials showing very high conductivities, several orders of magnitude higher than normal ionic solids
-
Conductivities greater than 10-2 (W-cm)-1 at temperatures much lower than melting points
-
Number of charge carriers is a very large fraction of potentially mobile ions which is significantly larger than in a normal ionic compound hence ion diffusion is solely governed by the diffusion energy and hence defect formation energy need not be provided resulting in overall low activation energy for migration.
Intrinsic FICs
-
Halides and Chalcogenides of silver and copper, e.g.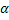 - AgI is a silver ion (Ag+ ) conductor - AgI is a silver ion (Ag+ ) conductor
-
Alkali metal conductors such as non-stoichiometric aluminates e.g. β-Al2O3 or Na2O.11Al2O3.
-
Extrinsic FICs
-
Oxides with fluorite structure such as ZrO2 are doped with aliovalent oxides creating a large number of oxygen vacancies and are called as fast ion conductors such as Y2O3.ZrO2. In these materials the enthalpy of migration, ΔHm , can be as low as ~ 0.01-0.2.
| Table 3.4: Examples of Fast Ion Conducting Ceramics and Conducting Ions |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
|
|
|
|