3.8 Ionic and Electronic Conductivity
Electrical conductivity (σi) is defined as charge flux per unit electric field with units (Ω-1cm-1) or S/m. It can be expressed as
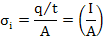 |
OR |
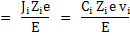 |
OR |
σi 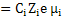 |
(3.39) |
where Ji is the flux of species i.
For ionic species, we can apply Nernst-Einstein equation, i.e. equation (3.29).
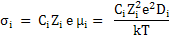 |
(3.40) |
Note:
As we saw earlier that temperature dependence of diffusivity shows two distinct regions: a low temperature extrinsic region and a high temperature intrinsic region. For the same reasons, temperature dependence of ionic conductivity also exhibit intrinsic and extrinsic regions. While in intrinsic regions, conductivity is governed only by defect migration as defect concentration is independent of temperature, in the extrinsic region, defect concentration is temperature dependent as well leading higher slope of log σ vs 1/T plot consisting of energy for defect creation as well as defect migration.
3.8.1 Total conductivity and Transference Number
Since all charged species contribute to the electrical conductivity, we can write total conductivity as
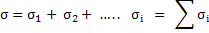 |
(3.41) |
Fraction of total conductivity carried by each charged species is called as transference number, ti and is expressed as
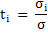 |
(3.42) |
and it is straightforward to see that
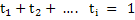 |
(3.43) |
Naturally when for a predominantly electronic conductor,
and for a predominantly ionic conductor
and for mixed conduction
As shown below, Table 3.2 lists the transference numbers for some conducting oxide ceramics.
Compound |
T(°C) |
|
|
|
ZrO2+7%CaO |
>700 |
0 |
1.0 |
10-4 |
Na2O.11Al2O3 |
<800 |
1 (Na+) |
-- |
<10-6 |
FeO |
800 |
SiO2 |
-- |
1.0 |
ZrO2+18%CeO2 |
1500 |
-- |
0.52 |
0.48 |
ZrO2+50%CeO2 |
|
-- |
0.15 |
0.85 |
Na2O.CaO.SiO2 Glass |
|
1 (Na+) |
- |
- |
15%(FeO.Fe2O3)CaO.SiO2.Al2O3 Glass |
1500 |
0.1 (Ca2+) |
- |
0.9 |
-
Typically ionic conductors exhibit a temperature dependence of mobility, i.e. mobility is thermally activated. Typically conductivities of ionic conductors are in the range of 10-5 to 100 S/m, depending on the temperature and are about 3 - 5 orders of magnitude lower than the metallic conductors but about 10 - 15 orders of magnitude higher than the ceramic insulators.
- Easily reducible oxides such as TiO2 , SnO2 , ZnO, BaTiO3 and SrTiO3 show an n-type semiconducting behaviour due to creation of oxygen vacancies and compensating electrons, whilst easily oxidizable oxides are CoO, FeO types which have cation deficiency and exhibit p–type behaviour.
|