2.19 Examples
2.19.1 Intrinsic electronic and ionic defect concentrations in MgO
Consider that a material like MgO usually has Schottky defects with enthalpy of formation (ΔHF)) of about 7.7 eV. Its band gap is about 7.65 eV which decreases at a rate of 1 meV per K as MgO is heated.
The question is that in case of an absolutely pure and stoichiometric MgO, which defects are likely to be created and present in higher concentrations at a temperature of say 1400°C or 1673 K?
We can calculate the Schottky defect concentration as
Electron and hole concentrations are calculated as
In MgO,
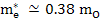 and and 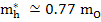 |
where mo is the mass of free electron and is 9.1×1031 kg.
At 1673K, Eg = 7.85 eV – (1570*1*103 ) eV = 6.28 eV .
Hence, ne = nh = 4.6*1010 cm3
Now magnesium vacancy concentration can be calculated as
Hence, at 1400°C, despite high energy of Schottky defect formation, the vacancy concentration will be slightly larger than the electronic carrier concentration due to thermal excitation.
|