2.17 Example: Comparative Behavior of TiO2 and MgO vis-à-vis Oxygen Pressure
Magnesium oxide in intrinsic form, primarily contains Schottky defects. However, under oxidizing conditions, defect reaction would be
with the equilibrium constant as 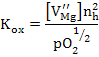 which is experimentally determined to be which is experimentally determined to be
It can be seen from the above equations that Kox increases as the temperature increases; i.e. MgO can be oxidized at high temperatures producing extrinsic Mg vacancies and holes if no other defects are present.
Further from electrical neutrality condition,
It should be noted that though actual concentrations can be very small but they still do vary with temperature and oxygen pressure.
For example, at 80% of Tm (2480 K) in air i.e. pO2 = 0.21 atm
which is equivalent to a deficiency of x=0.6 ppm in Mg1-xO,
While in pO2 of 10-9 MPa,
which is equivalent to a deficiency level of x=0.04 ppm in Mg1-xO.
In comparison, TiO2-x is more non-stoichiometric and prone to having oxygen deficiency. The reduction reaction is
From the electrical neutrality condition
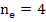 [ [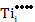 ] ] |
and |
The reaction constant is
and is experimentally determined to be 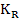 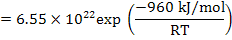 MPa.cm-1 . MPa.cm-1 .
Now, at 0.8 Tm, i.e. 1690 K
In air
[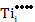 ] = ] = 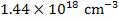 |
which is equivalent to a deficiency (x) of 93 ppm.
While at a pO2 of 10-9 MPa,
[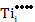 ] = ] = 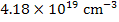 |
which is equivalent to a deficiency of x~0.27% in TiO2-x and makes TiO2-x an n-type semiconductor due to the resulting high electron concentration.
|