2.13 Defect Structures involving Oxygen Vacancies and Interstitials
Depending upon the partial pressure of oxygen, an oxide may be oxygen deficient (or metal excess) or metal deficient (or oxygen excess).
Let us consider the following conditions in an oxide MO:
-
Low pO2 i.e. oxygen vacancies dominate.
-
High pO2 i.e. oxygen interstitials dominate.
-
At intermediate pO2 i.e. oxide is stoichiometric.
Assuming that both oxygen vacancies and oxygen interstitials are doubly charged (fully ionized), the defect reactions can be written as follows:
The defect reaction can be written as
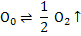 +
[ +
[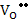 ] + 2e' ] + 2e' |
The corresponding reaction constant, assuming [MM] and [OO] =1, would be
The defect reaction is
and hence the reaction constant is
At intermediate pO2 Stoichiometric defects are likely to prevail i.e. either via intrinsic ionization or Anti-Frenkel defects.
Intrinsic ionization of electrons and holes
and corresponding reaction constant is
Similarly formation of oxygen Frenkel defects (Anti-) leads to
with reaction constant as
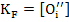 .[ 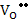 ] |
(2.53) |
From the above four relations, we can write
|